Abstract
Introduction: There has been significant debate on the best transfusion practices in cirrhotic patients with massive hemorrhage. As the liver is responsible for the synthesis of many proteins involved in coagulation and anticoagulation, cirrhosis can lead to multiple coagulation abnormalities. Portal hypertension, endothelial dysfunction, renal failure, and sepsis further contribute to increased bleeding tendency in cirrhosis. A growing area of interest is transfusion of cold-stored, low-titer Group O whole blood (LTOWB). The primary advantage of LTOWB versus component-based transfusion is that it provides a balanced resuscitation of all components that is closest to physiologic blood. Additionally, it contains lower quantities of anticoagulant and preservative solution compared to individual blood components. Previous studies suggest that LTOWB is at least equivalent if not superior to component therapy in resuscitation of life-threatening hemorrhage in trauma patients. The goal of the present study is to determine whether this positive effect is also observed in cirrhotic patients requiring activation of massive transfusion protocol (MTP) for non-traumatic bleeding.
Methods: We retrospectively collected data from cirrhotic patients receiving MTP with at least one unit of LTOWB transfusion between January 2019 and November 2021. LTOWB is obtained from the American Red Cross and leukoreduced with a platelet sparing filter from Terumo (Lakewood, CO). It is stored for up to 14 days, and if unused is subsequently converted to red blood cells. Low titer is defined as < 1:256. Baseline samples were collected just prior to initiation of LTOWB MTP. Post-transfusion samples were collected 24 hours following the activation of the MTP. We describe the degree of anemia, thrombocytopenia, coagulopathy, and hemolysis before and after LTOWB MTPs. The difference between post-transfusion total bilirubin and pre-transfusion total bilirubin was used as a surrogate for hemolysis. Pre- and post-transfusion thromboelastography (TEG) was used to assess clot formation, clot strength, and fibrinolysis. Data are reported as mean ± standard deviation for normally distributed variables, or median with 1st and 3rd quartiles if non-normal. The Wilcoxon signed rank test was employed to test changes between pre-and post-transfusion parameters (p<0.05 considered statistically significant).
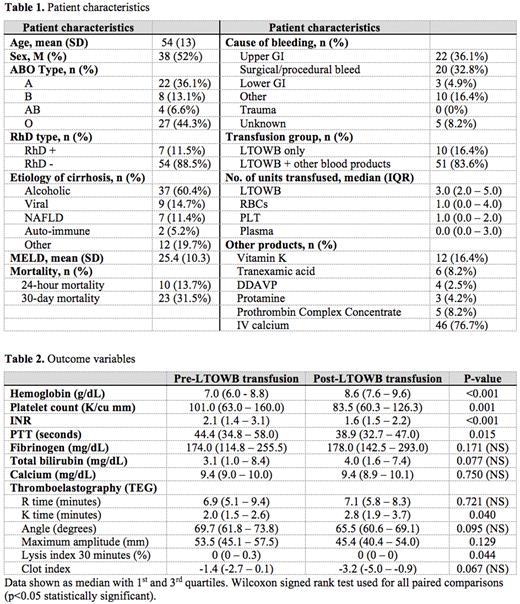
Results: Of 810 patients who received LTOWB MTP between January 2019 and November 2021, 317 were for non-traumatic bleeding, and of these 61 patients had cirrhosis. Table 1 describes patient characteristics. The most common etiology of cirrhosis was alcohol-associated liver disease. The most common cause of bleeding was upper GI bleed, followed by procedural bleeding. 16.4% of the patients received LTOWB only, and 83.6% of patients received both LTOWB and component therapy. On average, patients received one unit of red blood cells and one unit of platelets in addition to 3 units of LTOWB. Table 2 shows outcome variables. Of the 61 patients who received LTOWB MTP, Hemoglobin significantly increased 1.7 g/dL (P <0.001). Similarly, INR and PTT significantly decreased following LTOWB MTP (P <0.01 for both). Interestingly, there was a 17.5 K/cu mm decrease in platelet count (P = 0.001). The total bilirubin, calcium, and fibrinogen levels were unaffected by LTOWB MTP. The TEG K time was significantly prolonged following LTOWB MTP, while the lysis time at 30 minutes was decreased (P <0.05). One patient had a transfusion reaction.
Conclusions: To our knowledge, this is the first study examining LTOWB transfusion in non-trauma patients with cirrhosis. This proof of concept study suggests that LTOWB transfusion is a feasible option for this patient population. We observed a significant improvement in hemoglobin and PTT/INR, without observed signs of hemolysis. Our TEG results suggest decreased fibrinolysis following LTOWB MTP. An important limitation of this study is the lack of an appropriate control group. Ongoing work indicates that we will soon have enough data to compare our LTOWB versus component-only MTP cohorts.
Disclosures
Schreiber:Haemonetics: Consultancy, Research Funding; CSL Behring: Consultancy, Research Funding. Shatzel:Aronora, Inc.: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal